Don’t miss the whole story behind the ROVI’s Forest and the reforestation of the Valley of Dreams.
Don’t miss the video we have prepared for you. Click on the following image to watch it.

Laboratorios Farmacéuticos ROVI announces its incorporation as a member of the General Assembly of the Spanish Association of Biosimilar Medicines, BioSim. This is another milestone for both entities.
Laboratorios Farmacéuticos ROVI is a group of the pharmaceutical sector of Spanish origin, specialized, fully integrated and dedicated to the research, development, manufacture under license and commercialization of small molecules and biological specialties. Since 2017, the group has boosted its international presence thanks to the commercialization of its enoxaparin biosimilar. For ROVI, joining Biosim means joining “the work of an essential actor in the dissemination of knowledge and the promotion of biosimilar medicines in Spain, quality therapeutic alternatives that contribute to the sustainability of the health system”.
For BioSim, for its part, the incorporation of ROVI represents an advance in the representativeness of the Association as a reference for laboratories that research, develop, produce and/or market biosimilar medicines, with common principles and objectives. Thus, through different initiatives, both organizations will continue to promote the use of biosimilar medicines and transfer their value to the administration, health professionals, patients and the general public.
In the words of Encarna Cruz, CEO of BioSim “ROVI, as one of the pioneering companies that develops and produces biosimilars in Spain and markets internationally, is an example of a company that bets and encourages national employment and biosimilars. From BioSim we thank you for joining the Association and we believe that it can enrich the debates and decisions that are taken from now on. ”
This incorporation, in addition, adds the president of BioSim, Joaquín Rodrigo, increases the representativeness of the Association that currently has companies that occupy more than 95% of the Spanish biosimilar market. This allows us to strengthen our role as an interlocutor before administrations, managers, health professionals and patients. With its incorporation, the company wants to contribute its experience in the bioisimilars market. “Our enoxaparin biosimilar has become one of the main value generators of the group. We started its commercialization in 2017 and, since then, it has been a great boost to ROVI’s internationalization strategy and we hope that it will continue to be a lever of growth for our company in the coming years, ”explains Pedro Carretero, director of Hospitals and Institutional Relations of ROVI Laboratories and representative of the group in Biosim.
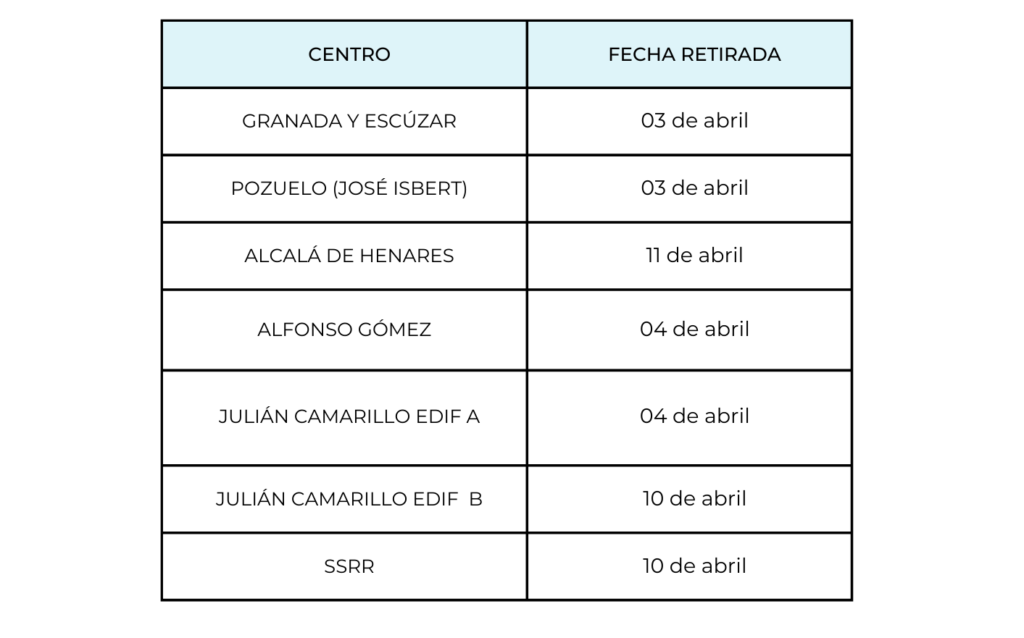
To improve the vending service, a change of supplier has been made from Selecta to Alliance Vending.
Due to this change, the old cards will be withdrawn and the new ones will be issued. For this reason, it is recommended that the balance of the old cards be used up before the withdrawal deadlines:
The new vending machines will be located in the canteen areas of each headquarters. We hope you enjoy them.
ROVI presented its ISM® technology platform at the meeting of the Innovation Committee of the Institute for the Development and Integration of Healthcare (IDIS Foundation). The debate was attended by Pedro Carretero Trillo, Director of Hospitals and Institutional Relations; Ibon Gutierro Adúriz, Director of R&D, and Javier Martínez González, Medical Director.
Throughout the colloquium, a presentation was made showing the advances throughout history in relation to long-acting injectables and the importance of achieving a reduction in symptomatology as quickly as oral treatment. In this sense, the results of ISM® Technology, designed to combine the sequential effect of three different mechanisms to control the release of active ingredients, were highlighted, as it provides therapeutic levels as fast as oral medication and maintains them in a sustained and predictable way.