Laboratorios Farmacéuticos Rovi, S.A. has held its Ordinary General Shareholders’ Meeting, at which it presented an evaluation of the financial year 2023 and its plan for the launch of Risperidone ISM® for Europe and the rest of the world, with which it hopes to achieve potential sales of between 200 and 300 million euros globally in upcoming years.
The Meeting, with quorum on the first call, has passed all the motions on the Agenda that are submitted for its approval, including, among others, the motion to approve the individual and consolidated annual accounts of the company and the respective management reports for the year ended 31 December 2023, as well as the corporate management during the last financial year.
The company has presented its shareholders with consolidated operating revenue of 829.6 million euros in 2023, representing 1% growth on 2022, demonstrating the resilience of its business in the first year of a new endemic scenario.
CDMO sales rose 1% in 2023 compared with the preceding year 2022, totalling 409.3 million euros in 2023, mainly due to: (i) the booking of the revenue related to production of the COVID-19 vaccine; (ii) the booking of the revenue related to the activities carried out to prepare the plant to produce the vaccine under the agreement with Moderna; and (iii) the reorientation of the CDMO strategy towards products with a higher value-added.
The specialty pharmaceutical business sales increased 1% in 2023 compared to 2022, totalling 420.2 million euros, mainly driven by Okedi®, Neparvis® and Orvatez®.
Sales of Okedi®, ROVI’S first product based on its vanguard drug delivery technology, ISM®, indicated for the treatment schizophrenia in adults for whom tolerability and effectiveness have been established with oral risperidone, were 14.4 million euros in 2023. These sales increased 42% in the fourth quarter of 2023 in comparison with the third quarter of the year. In 2022, the product was launched in Germany, the United Kingdom and Spain and, in 2023, in Portugal, Italy, Austria, Greece and Serbia. It is important to highlight the fact that, in the first quarter of 2024, the product was approved in the United States (under the trademark Risvan®), Canada and Australia.
Sales of Neparvis® grew 16% to 45.5 million euros, compared to 39.1 million euros in 2022. This is a prescription product from Novartis indicated for the treatment of adult patients with symptomatic chronic heart failure and reduced ejection fraction, which ROVI has been distributing in Spain since December 2016.
Sales of Orvatez®, a prescription product from the company Organon & Co. (“Organon”) indicated as adjunctive therapy to diet in patients with hypercholesterolemia, increased 8% to 26.5 million euros in 2023, compared with the 24.5 million euros of 2022.
Regarding the heparin franchise, ROVI aspires to become a global leader in the low-molecular-weight heparin (“LMWH”) field. The commitment to this franchise can be seen with the inauguration, in the fourth quarter of 2023, of a new production plant for the active substance of heparins, and the addition, in the second quarter of 2024, of a new sodium heparin facility, both of which are in Escúzar (Granada). Likewise, the company continues with its commitment to the vertical integration of the heparin supply chain by investing in the creation of the first national structure for the self-supply of low-molecular-weight heparins. ROVI expects this investment to contribute to an increase in the future margins of the heparin franchise and believes that the plant is likely to come into operation in 2026. Additionally, LMWH raw material prices dropped 35% in 2023 compared with 2022. This decrease is expected to accelerate during 2024 and to have a positive impact on the group’s gross margin from 2025 onwards.
In 2023, LMWH sales decreased 6% on 2022, dropping to 242.1 million euros in 2023, mainly as a result of the difference between the increase in partners’ orders related to COVID-19 treatment in 2022 and the lower volume of orders from partners in 2023, since they had held higher stocks of the products since 2022.
EBITDA decreased 12% on 2022 to 244.5 million euros in 2023, reflecting a drop of 4.6 percentage points in the EBITDA margin, which was 29.5% in 2023, compared to 34.1% in 2022.
Net profit totalled 170.3 million euros in 2023, showing a 15% decrease on the 2022 figure, which had been 199.7 million euros.
Net profit totalled 170.3 million euros in 2023, with a 15% decrease on the 2022 figure, which was 199.7 million euros.
Research and development (R&D) expenses increased 4% to 24.9 million euros in 2023. These R&D expenses relate mainly to (i) developing the phase 1 of Letrozole LEBE and (ii) developing the phase I of the new formulation of Risperidone ISM® for a three-monthly injection.
Sales, overhead and administration expenses rose 2% to 219.7 million euros in 2023, mainly resulting from the increase in expenses related to the launch of Okedi® in Europe. Notwithstanding, other operating expenses (excluding R&D and employee benefit expenses) decreased 11% to 106.4 million euros in 2023, due to an efficient cost containment policy.
Consolidation and international expansion plan for Risperidone ISM® in Europe and the rest of the world
The European Commission authorised the marketing of Okedi® (Risperidone ISM®) in February 2022. Since then, the product has been marketed in a number of European countries. In 2022, Okedi® was launched in Germany, the United Kingdom and Spain and, in 2023, in Portugal, Italy, Austria, Greece and Serbia. These launches have consolidated ROVI’s internationalisation strategy as one of its pillars for future growth.

Furthermore, in March 2024, the FDA approved the marketing of Risvan® (Risperidone ISM®) for the treatment of schizophrenia in adults. Additionally, the FDA has required, as a postmarketing requirement following normal market practice, that a pharmacokinetic study be conducted to evaluate exposure to Risvan® similar to the daily administration of 6mg of oral risperidone. The protocol for the clinical study will be previously reviewed and agreed with the FDA and the final report on the study will be submitted before July 2026. This additional study does not affect either the approval or the marketing of Risvan®. Likewise, ROVI remains committed to the company’s international expansion outside Europe as a result of the approval of this product in Canada and Australia, also in 2024.
ROVI expects that, given its differential characteristics, Risperidone ISM® will reach potential sales of between 200 and 300 million euros globally in upcoming years and will become a significant player worldwide in the field of long-acting injectables to treat schizophrenia.
Expansion of ROVI’s industrial presence
In June 2024, ROVI has obtained the European authorities’ approval for the commencement of commercial activity at its new sodium heparin plant in Escúzar (Granada). Thus, ROVI is positioned as one of the largest pharmaceutical industrial groups in Spain, with eight fully-integrated plants and a ninth under construction.
The Group has five plants to manufacture its own products and three for contract manufacturing. In Andalusia, it has three plants for its own manufacturing: two engaged in producing the active substance of low-molecular-weight heparins, in Granada and Escúzar, and the new plant that will be producing sodium heparin. ROVI is, therefore, prepared for production of a medicine like sodium heparin, which is classified as essential by the World Health Organisation and is, moreover, among the medicines included in the European Union’s Critical Medicine Alliance, in which ROVI participates.
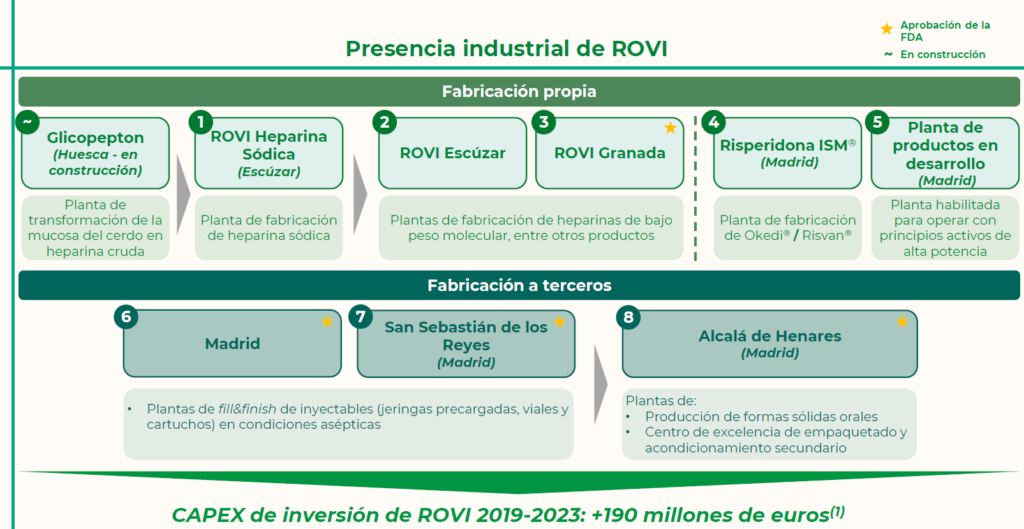
Additionally, ROVI has two plants in Madrid engaged in the production of medicines based on its ISM® technology, in which 35.6 million euros has been invested in the last five years: at the first plant, the company produces Risperidone ISM®, while the second is used to manufacture products under development that use highly potent active ingredients.
Furthermore, ROVI has three plants engaged in contract manufacturing: in particular, two injectables manufacturing plants, located in San Sebastián de los Reyes and Madrid, and a third in Alcalá de Henares, which is engaged in producing solid oral forms and is a packaging centre of excellence.
Likewise, ROVI remains committed to the vertical integration of its value chain in order to achieve strategic autonomy in its medicine manufacturing process. In this respect, ROVI is making significant investments in the construction of a new plant in Huesca, which will transform pig mucosa into crude heparin and is considered likely to come into operation in 2026.





